




 What are the Alkali Metals?
What are the Alkali Metals?
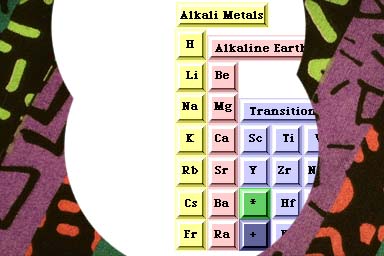 The Alkali Metals are the first family of the Periodic Table.
They have one electron in their outer orbitals.
In a chemical reaction, this outer electron is lost to form an ion with a single positive charge.
The term alkali means basic with pH greater than 7 as opposed to acids which are less than 7.
When these Metals combine with Non-Metals, the result is a base.
Alkali Metals are silver-white, malleable, soft, and soluble in water.
An Alkali Metal isolated in nature is rare because it reacts so readily with many Non-Metals especially the Halogens.
[LM]
{CI}
The Alkali Metals are the first family of the Periodic Table.
They have one electron in their outer orbitals.
In a chemical reaction, this outer electron is lost to form an ion with a single positive charge.
The term alkali means basic with pH greater than 7 as opposed to acids which are less than 7.
When these Metals combine with Non-Metals, the result is a base.
Alkali Metals are silver-white, malleable, soft, and soluble in water.
An Alkali Metal isolated in nature is rare because it reacts so readily with many Non-Metals especially the Halogens.
[LM]
{CI}
 Web Pages that Work! Zeuter Development Corporation
Web Pages that Work! Zeuter Development Corporation
Post Office Box 225, Parry Sound, Ontario, CANADA P2A 2X3
Copyright © Zeuter Development Corporation, 1996-2022. All rights reserved.
 The Alkali Metals are the first family of the Periodic Table.
They have one electron in their outer orbitals.
In a chemical reaction, this outer electron is lost to form an ion with a single positive charge.
The term alkali means basic with pH greater than 7 as opposed to acids which are less than 7.
When these Metals combine with Non-Metals, the result is a base.
Alkali Metals are silver-white, malleable, soft, and soluble in water.
An Alkali Metal isolated in nature is rare because it reacts so readily with many Non-Metals especially the Halogens.
[LM]
{CI}
The Alkali Metals are the first family of the Periodic Table.
They have one electron in their outer orbitals.
In a chemical reaction, this outer electron is lost to form an ion with a single positive charge.
The term alkali means basic with pH greater than 7 as opposed to acids which are less than 7.
When these Metals combine with Non-Metals, the result is a base.
Alkali Metals are silver-white, malleable, soft, and soluble in water.
An Alkali Metal isolated in nature is rare because it reacts so readily with many Non-Metals especially the Halogens.
[LM]
{CI}